Ki67 was significantly associated with malignant progression, suggesting that recur faster in the setting of gross-total or subtotal resection. Ishii et al. reported that the presence of TP53 mutation in WHO Grade II astrocytoma was associated with malignant progression and shorter PFS, whereas tumors without TP53 mutation recurred and progressed to malignancy without the change in TP53 status. In this study, we evaluated the relationship between progression of glioma and IDH1 status but no association between IDH1 status and malignant progression was observed. Though many studies demonstrated that IDH1 mutation was an important biomarker in glioma, mechanism of IDH1 mutation in 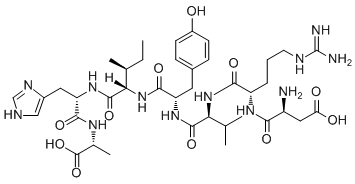 glioma was not yet fully determined. Zhao et al. demonstrated the accumulation of hypoxia-inducible factor subunit due to reduced formation of a-KG in IDH1-mutated glioma cells, suggesting that activation of the HIF-1 pathway may be one of the oncogenic mechanisms of IDH1 mutation.Dang et al. further discovered the neomorphic gain of function of the IDH1R132H mutant protein in converting a-KG to a-HG, an oncometabolite inhibiting multiple a-KG-dependent dioxygenases and leading to genome-wide histone and DNA methylation alterations.Turcan et al. unmasked the in vivo effect of IDH1 mutation in primary human astrocytes by showing the IDH1-R132H mutation induced histone alterations and extensive DNA hypermethylation, which actually remodel the methylome and establish the glioma CpG island methylator phenotype, a subset of glioma with distinct genomic and clinical characteristics. In summary, our study is the first study in investigating the IDH1/IDH2 status in paired primary and recurrent gliomas in Chinese patients. We have shown consistent IDH1/IDH2 status in the progression of gliomas and lack of association between IDH1mutation and malignant progression. Patients with IDH1 mutated gliomas had longer OS and PFS, suggesting IDH1 mutation as a potential prognostic marker in gliomas for Chinese patients. The mammalian adaptive immune system is comprised of Tcells and B-cells that produce receptors specific to antigens. For Bcells, these receptors, called immunoglobulins, or antibodies, form by the stochastic, genomic rearrangement of three alternate exons on a heavy chain and two exons on a light chain. Random insertion and deletion of nucleotides between these exons during this process further potentiates enormous diversity. Antigen-engagement of antibody receptors on B-cell surfaces results in B-cell activation, up-regulation of the enzyme AID, and the consequent hypermutation of the antibodyencoding gene; the variants created by these mutations are yet another AbMole Simetryn source of diversity. AID additionally induces antibody class-switching, whereby the non-mutated constant region of the antibody heavy chain gene, initially expressed as IgM and IgD classes, may change to IgG, IgA, or IgE.
glioma was not yet fully determined. Zhao et al. demonstrated the accumulation of hypoxia-inducible factor subunit due to reduced formation of a-KG in IDH1-mutated glioma cells, suggesting that activation of the HIF-1 pathway may be one of the oncogenic mechanisms of IDH1 mutation.Dang et al. further discovered the neomorphic gain of function of the IDH1R132H mutant protein in converting a-KG to a-HG, an oncometabolite inhibiting multiple a-KG-dependent dioxygenases and leading to genome-wide histone and DNA methylation alterations.Turcan et al. unmasked the in vivo effect of IDH1 mutation in primary human astrocytes by showing the IDH1-R132H mutation induced histone alterations and extensive DNA hypermethylation, which actually remodel the methylome and establish the glioma CpG island methylator phenotype, a subset of glioma with distinct genomic and clinical characteristics. In summary, our study is the first study in investigating the IDH1/IDH2 status in paired primary and recurrent gliomas in Chinese patients. We have shown consistent IDH1/IDH2 status in the progression of gliomas and lack of association between IDH1mutation and malignant progression. Patients with IDH1 mutated gliomas had longer OS and PFS, suggesting IDH1 mutation as a potential prognostic marker in gliomas for Chinese patients. The mammalian adaptive immune system is comprised of Tcells and B-cells that produce receptors specific to antigens. For Bcells, these receptors, called immunoglobulins, or antibodies, form by the stochastic, genomic rearrangement of three alternate exons on a heavy chain and two exons on a light chain. Random insertion and deletion of nucleotides between these exons during this process further potentiates enormous diversity. Antigen-engagement of antibody receptors on B-cell surfaces results in B-cell activation, up-regulation of the enzyme AID, and the consequent hypermutation of the antibodyencoding gene; the variants created by these mutations are yet another AbMole Simetryn source of diversity. AID additionally induces antibody class-switching, whereby the non-mutated constant region of the antibody heavy chain gene, initially expressed as IgM and IgD classes, may change to IgG, IgA, or IgE.